Celestine (4.22 ct) from Madagascar
- Details
- Created: Saturday, 03 June 2023 11:05
Initially published as a Gem note in the Journal Of Gemmology of the Gem-A. 2020, 37(4), 344–346.
http://doi.org/10.15506/JoG.2020.37.4.344
Celestine (or celestite, coelestite) is named after the Latin caelestis, alluding to the colour of the sky. It is a strontium sulphate (SrSO4) of the baryte group, where the other isostructural members are baryte (BaSO4), anglesite (PbSO4) and anhydrite (CaSO4). A solid-solution series exists between celestine and baryte, and the name baritocelestine is sometimes used for barium-rich celestine. Celestine is colourless to pale blue, although rare orange material has been found in Ontario, Canada (Bernstein 1979[1]). Although celestine occurs worldwide, Madagascar is the main source and has produced many hundreds of tons of geodes showing pale blue colour, including the world’s finest specimens. The mining area is located north and west of Sakoany village in the Mahajanga (Majunga) region on the north-western coast of Madagascar, near the mouth of Betsiboka River (Pezzotta 1999[2]; Wilson 2010[3]; Pezzotta & Pezzotta 2020[4]). Celestine is most commonly seen on the market as tumbled stones and mineral specimens, and is sometimes fashioned into cabochons. It is rarely encountered as faceted stones; its low hardness (Mohs 3–3½), perfect cleavage and brittleness make it difficult to facet.
 Figure 1. This 4.22 ct celestine (9.4 × 7.6 × 6.4 mm) is reportedly
Figure 1. This 4.22 ct celestine (9.4 × 7.6 × 6.4 mm) is reportedlyfrom Madagascar. It was fashioned by Ottorino Invernizzi from a
crystal taken from a mineral specimen.
The 4.22 ct faceted celestine in Figure 1 is reportedly from Madagascar and was characterised by the author to add more gemmological data to the literature on this collector’s stone. It showed the following properties (Table 1). These characteristics are consistent with those previously reported for celestine (O’Donoghue 2006[5]). Microscopic observation (up to 40×) showed no visible inclusions.
| Shape | rectangular mixed cut |
| Size | 9.4 x 7.6 x 6.4 mm |
| Color | almost colourless with a very faint greyish blue tint |
| Lustre | vitreous |
| Diaphaneity | transparent |
| Weight | 4.22 |
| SG | 3.98 |
| RI | 1.620–1.631 |
| DR | 0.011, biaxial positive |
| Pleochroism | light green-blue, light bluish violet and colourless |
| Polariscope / Conoscope | - |
| SWUV | inert |
| LWUV | inert |
| Magnetic susceptibility N52 | diamagnetic (repelled by magnet); |
| Chelsea filter | none |
Table 1. Observational and measured properties
Infrared reflectance spectroscopy:
Infrared (IR) reflectance spectra were collected from several spots on the stone’s table and pavilion facets, and a representative spectrum is provided in Figure 2. Although the anisotropy of the material significantly modified the pattern in the 1300–1000 cm–1 range, overall the data matched the reference spectrum published by Hainschwang and Notari (2008)[6]. It is possible to distinguish celestine from baryte using IR spectroscopy because the ν1 band (stretching mode) is at 991 cm–1, versus 981 cm–1 for baryte. Three bands (ν4) at 614–618, 643 and 654 cm–1 for celestine (compared to 610–614, 635 and 648 cm–1 for baryte) can also be used, but the patterns of these bands are sensitive to anisotropy. The bands’ assignments were reported by Lane (2007)[7].
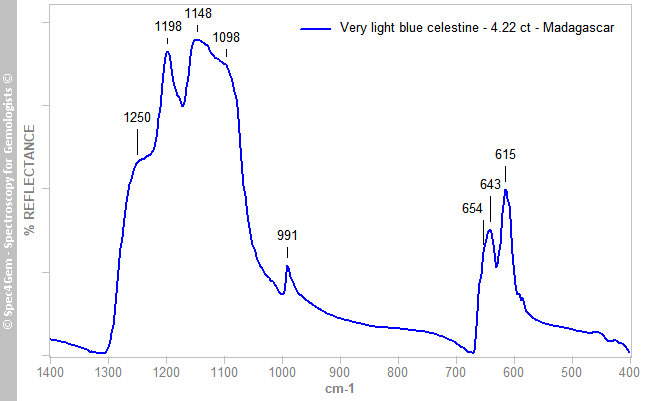 Figure 2. The IR reflectance spectrum of celestine is characterised by bands in the ranges 1200–1080 and 680–580 cm–1, as well as a ν1 band at 991 cm–1.
Figure 2. The IR reflectance spectrum of celestine is characterised by bands in the ranges 1200–1080 and 680–580 cm–1, as well as a ν1 band at 991 cm–1.UV-VIS-NIR spectroscopy:
Visible-range spectra (Figure 3) were collected for each polarisation direction corresponding to the three pleochroic colours, and the path lengths were used to calculate absorption coefficients. The E||b spectrum (green-blue) consisted of two broad bands at 425 and 618 nm that created a transmission window at about 510 nm. The E||c spectrum (bluish violet) was similar to E||b, but one of the broad bands (605 nm) was somewhat shifted towards the blue region and it lacked the 425 nm band. An asymmetric band (shoulder) on the low-wavelength side of the 605–618 nm absorption is due to an underlying band at around 575–585 nm. The E||a spectrum (colourless) was rather flat.
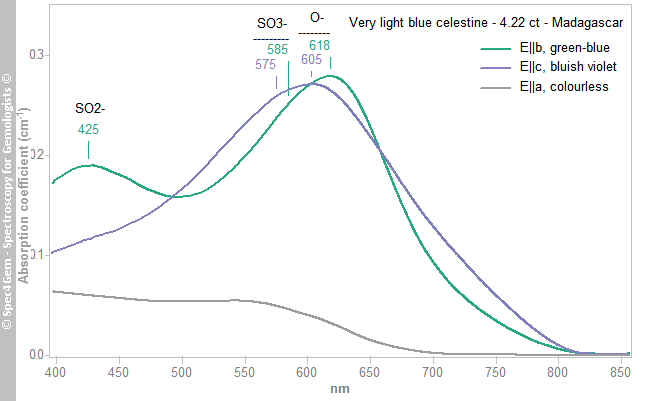 Figure 3. Polarised visible-range spectra are shown for the green-blue (E||b), bluish violet (E||c) and colourless (E||a) pleochroic directions of the 4.22 ct celestine. The major absorption bands are due to O–, SO3– and SO2–.
Figure 3. Polarised visible-range spectra are shown for the green-blue (E||b), bluish violet (E||c) and colourless (E||a) pleochroic directions of the 4.22 ct celestine. The major absorption bands are due to O–, SO3– and SO2–.The causes of colour in celestine were studied with EPR spectroscopy by Bernstein (1979), who reported the presence of colour centres due to radiation damage; no more recent work appears to have been published. Bernstein (1979)[1] indicated that the 425 nm band is related to SO2– , the 575–585 nm band might be due to SO3– and the 605–618 nm feature is associated with O–. These centres are stabilised by the presence of trace components, primarily K+ substituting for Sr2+. Bernstein (1979)[1] also performed heat-treatment experiments and found that thermal bleaching occurred at just 200°C for heating durations of a minute to several days; the colour could be restored by irradiation
Photoluminescence spectroscopy:
Celestine has been reported in the literature to be sometimes ‘fluorescent’, with a white, blue or even red reaction to UV radiation. A broad photoluminescence (PL) emission band usually peaking between 430 and 500 nm has been possibly connected to Pb2+ centres, but this has not been confirmed (Gaft et al. 2015[8]). Other studies have proposed several other possible luminescence causes, such as organic impurities and Eu2+ (Tarashchan 1978[9]), and at low temperature (77 K), O2–, VO4, TiO4 and MoO4 (Gaft et al. 1985[10]). The stone described here was analysed by PL spectroscopy using 254, 375 and 405 nm excitations at room temperature, but no photoluminescence was detected.
[1] Bernstein, L.R. 1979. Coloring mechanisms in celestite. American Mineralogist, 64(1–2), 160–168.
[2] Pezzotta, F. 1999. Heavenly: Madagascar’s celestite. In: Madagascar—A Mineral and Gemstone Paradise. ExtraLapis English No. 1, Christian Weise Verlag, Munich, Germany, and Lapis International, East Hampton, Connecticut, USA, 88–89.
[3] Wilson, W.E. 2010. The Sakoany celestine deposit, Mahajanga Province, Madagascar. Mineralogical Record, 41(5), 405–416.
[4] Pezzotta, F. & Pezzotta, S. 2020. La celestina, un classico della mineralogia del Madagascar. Rivista Mineralogica Italiana, 44(2), 94–108 (in Italian).
[5] O’Donoghue, M. 2006. Gems. Butterworth-Heinemann, Oxford, xxix + 873 pp.
[6] Hainschwang, T. & Notari, F. 2008. Specular reflectance infrared spectroscopy — A review and update of a little exploited method for gem identification. Journal of Gemmology, 31(1), 23–29, https://doi.org/10.15506/JoG.2008.31.1.23.
[7] Lane, M.D. 2007. Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals. American Mineralogist, 92(1), 1–18, https://doi.org/10.2138/am.2007.2170.
[8] Gaft, M.L., Bershov, L.V., Krasnaya, A.R. & Yaskolko, V.Y. 1985. Luminescence centers in anhydrite, barite, celestite and their synthesized analogs. Physics and Chemistry of Minerals, 11(6), 255–260, https://doi.org/10.1007/bf00307403.
[9] Tarashchan, A.N. 1978. Luminestsentsia Mineralov [Luminescence of Minerals]. Naukova Dumka, Kiev, Ukraine, 296 pp. (in Russian).
[10] Gaft, M., Reisfeld, R. & Panczer, G. 2015. Modern Luminescence Spectroscopy of Minerals and Materials. Springer, Cham, Switzerland, xix + 606 pp., https://doi.org/10.1007/978-3-319-24765-6.

