Crocoite (0.35 ct) from Tasmania, Australia
- Details
- Created: Monday, 29 May 2023 20:59
Initially published as a Gem note in the Journal Of Gemmology of the Gem-A. 2022, 38(1), 9–11.
http://doi.org/10.15506/JoG.2022.38.1.9
Crocoite (PbCrO4) was first found in Russia in 1763, later in a few European localities, and then in Australia (Tasmania) and Brazil. At one time it was the main ore of chromium, and it has also been used as a yellow pigment, such as in Van Gogh’s paintings and on American school buses. To the mineral collector, Tasmanian crocoite surpasses others because the specimens occur as well-formed crystals, and they are larger and more abundant than at any other locality. The (monoclinic) crystals are elongated and prismatic, and almost always vertically striated with a nearly square outline. They most often form reticulated aggregates and acicular groups; less commonly they occur as individual prismatic crystals that are sometimes partially hollow. Although crocoite possesses a remarkably saturated orange to orange-red colour, it is unknown to most gemmologists since its low hardness (Mohs 2½ –3) relegates it to a collectors’ stone. Crocoite’s colour can fade to brown or even black over a long period (i.e. a century) if exposed to light, as observed in Van Gogh’s paintings (Tan et al. 2013[1]) and in some mineral specimens (Grguric 2015[2]).
The 0.35 ct crocoite gemstone described here (Figure 1) is reportedly from Tasmania (i.e. possibly from the Adelaide or Red Lead Mines in Dundas, Zeehan District).
 Figure 1. This 0.35 ct crocoite (3.9 × 2.8 × 2.1 mm), reportedly from
Figure 1. This 0.35 ct crocoite (3.9 × 2.8 × 2.1 mm), reportedly fromTasmania, exhibits an attractive vivid orange colour. Near the
bottom, a partially healed fracture is visible as a dark elongated
area.
| Shape | rectangular step cut |
| Size | 3.9 x 2.8 x 2.1 mm |
| Color | orange |
| Lustre | vitreous to sub-adamantine |
| Diaphaneity | transparent |
| Weight | 0.35 ct |
| SG | 6.0 |
| RI | OTL |
| DR | strong (estimated by facet-edge doubling as 0.35) |
| Pleochroism | weak orange and orange-red (sometimes described as trichroic since the mineral is biaxial) |
| Polariscope / Conoscope | - |
| SWUV | inert |
| LWUV | inert |
| Magnetic susceptibility N52 | inert |
| Chelsea filter | inert |
Table 1. Observational and measured properties
These data (Table 1) are consistent with those previously published for crocoite (O’Donoghue 2006[3], who also reported RIs of 2.29–2.66 and a biaxial positive optic character).
Microscopic examination revealed long thin growth tubes aligned parallel to the stone’s length (likely the prism axis), very small negative crystals and a reflective partially healed fracture.
Infrared reflectance spectroscopy:
Infrared reflectance spectra were collected from several orientations of the stone’s table, and all yielded essentially the same pattern (Figure 2), which is comparable with published spectra of crocoite from Russia (Chukanov 2014[4]; Cr6 sample after log(1/T) transformation) and Tasmania (Crocoite database).
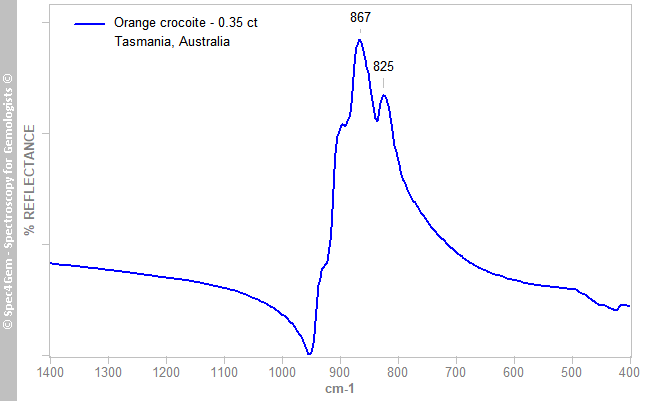 Figure 2. The IR reflectance spectrum of the stone is representative of crocoite, and is characteristic of a chromate anion with dominant bands due to ν3 asymmetric stretching vibrations in the 800–950 cm–1 range (Campbell 1965[9]).
Figure 2. The IR reflectance spectrum of the stone is representative of crocoite, and is characteristic of a chromate anion with dominant bands due to ν3 asymmetric stretching vibrations in the 800–950 cm–1 range (Campbell 1965[9]).Raman spectroscopy:
A Raman spectrum (Figure 3) was collected from the gem’s table with a homemade Raman spectrometer using a 638 nm LED laser without any polarisation consideration. The resulting spectrum was consistent with published crocoite spectra (e.g. Wilkins 1971[5]; RRUFF R160016).
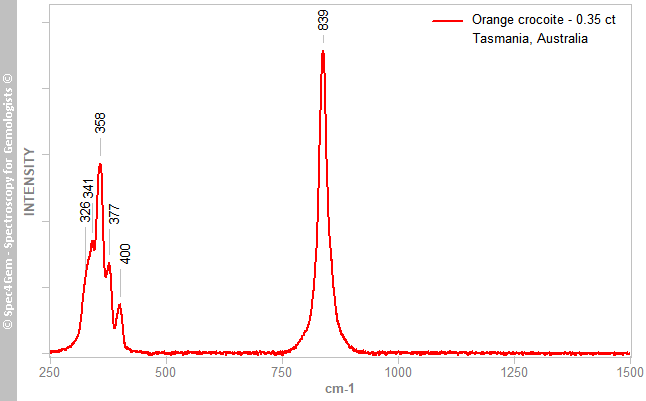 Figure 3. The Raman spectrum of the stone is also representative of crocoite. The bands at 326, 341, 358, 377 and 400 cm–1 are produced by ν2 and ν4 bending vibrations, while the 839 cm–1 (multicomponent) band results from ν1 and ν3 stretching vibrations in the CrO42– anion (Wilkins 1971[5]).
Figure 3. The Raman spectrum of the stone is also representative of crocoite. The bands at 326, 341, 358, 377 and 400 cm–1 are produced by ν2 and ν4 bending vibrations, while the 839 cm–1 (multicomponent) band results from ν1 and ν3 stretching vibrations in the CrO42– anion (Wilkins 1971[5]).UV-VIS-NIR spectroscopy:
Visible-near infrared (Vis-NIR) spectra were collected over the 360–1000 nm range (Figure 4; cropped to 400–800 nm) with the help of a polarising filter oriented to obtain the maxima corresponding to the observed pleochroic colours: orange and orange-red. The two spectra showed very strong absorption in the violet, blue and green regions, with a remarkably steep absorption edge between 570 and 620 nm (the strength of which surpassed the capacities of the spectrophotometer), and no other features above 650 nm.
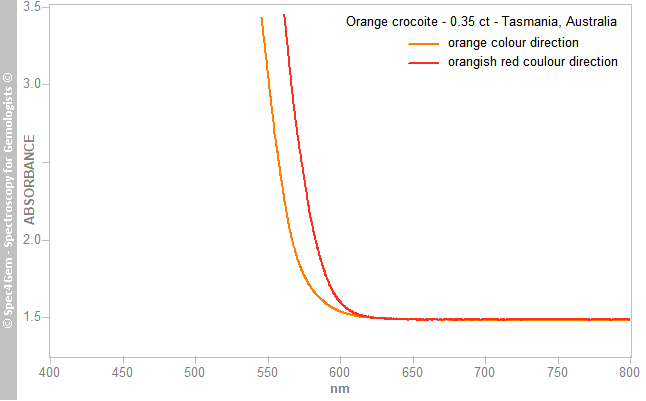 Figure 4. Polarised Vis-NIR spectra of the crocoite display a strong absorption over the violet-to-yellow range, with a remarkably steep absorption edge causing the orange and orange-red pleochroic colours. The path length of the beam was about 2.0 mm
Figure 4. Polarised Vis-NIR spectra of the crocoite display a strong absorption over the violet-to-yellow range, with a remarkably steep absorption edge causing the orange and orange-red pleochroic colours. The path length of the beam was about 2.0 mmThe absorption edge bordered, respectively, the transmission in the yellow and the red regions, which explains the observed colours; the shift between the two represents the pleochroism. Such a strong absorption is often associated with oxygen-metal charge transfer, in this case O2→Cr6+ within the CrO42– anion (Loeffler & Burns 1976[6]). Unlike some published spectra (Reddy & Sarma 1981[7]), there was no band at about 605 nm.
Photoluminescence spectroscopy:
Crocoite has sometimes been described as fluorescent (O’Donoghue 2006[3]) or as showing deep red luminescence, with a broad band peaking at about 700 nm (RFUFF R040053, 532 nm broad-scan spectrum) or 750 nm (with 337 nm excitation at liquid-nitrogen temperature; Gorobets & Rogojine 2002[8]). Photoluminescence spectra of the present sample were collected using 254, 280, 375, 405 and 532 nm excitations, but none showed any luminescence. Similarly, none was observed in the Raman spectrum.
Safety note:
Crocoite is toxic, as it contains both lead and hexavalent chromium, so it is important to avoid breathing its dust and to wash hands after handling it.
[1] Tan, H., Tian, H., Verbeeck, J., Monico, L., Janssens, K. & Van Tendeloo, G. 2013. Nanoscale investigation of the degradation mechanism of a historical chrome yellow paint by quantitative electron energy loss spectroscopy mapping of chromium species. Angewandte Chemie International Edition, 52(43), 11360–11363, https://doi.org/10.1002/anie.201305753.
[2] Grguric, B. 2015. The light sensitivity of crocoite. https://www.mindat.org/article.php/2252/The+Light+Sensitivity+of+Crocoite, accessed 3 February 2022.
[3] O’Donoghue, M. (ed) 2006. Gems. Butterworth-Heinemann, Oxford, 873 pp.
[4] Chukanov, N.V. 2014. Infrared Spectra of Mineral Species. Springer, Dordrecht, Germany, 1726 pp., https://doi.org/10.1007/978-94-007-7128-4.
[5] Wilkins, R.W.T. 1971. The Raman spectrum of crocoite. Mineralogical Magazine, 38(294), 249–250, https://doi.org/10.1180/minmag.1971.038.294.15.
[6] Loeffler, B.M. & Burns, R.G. 1976. Shedding light on the color of gems and minerals. American Scientist, 64(6), 636–647, https://www.jstor.org/stable/27847555.
[7] Reddy, B.J. & Sarma, K.B.N. 1981. Optical properties of chromate centres in crocoite. Physics Letters A, 86(6–7), 386–388, https://doi.org/10.1016/0375-9601(81)90564-8.
[8] Gorobets, B.S. & Rogojine, A.A. 2002. Luminescent Spectra of Minerals. RCP VIMS, Moscow, Russia, ISBN: 5-901837-05-3, 300 pp.
[9] Campbell, J.A. 1965. Spectral evidence for interionic forces in crystals—chromates and dichromates. Spectrochimica Acta, 21(7), 1333–1343, https://doi.org/10.1016/0371-1951(65)80215-6.

