Very-light-yellow willemite from Franklin, New Jersey, USA
- Details
- Created: Sunday, 13 January 2019 13:44
Willemite is a zinc silicate (Zn2SiO4) and forms stout and slender prismatic trigonal crystals or coarse to fine granular masses. The colors are colorless, yellow, yellowish-green,blue-green, brown and white. It occurs in zinc deposits in limestones and is an uncommon ore of zinc. Willemite is popular among minerals collectors because of its strong green fluorescence under short-wave ultraviolet light especially when combined with the red fluorescence of zincite and pink one of calcite.
Gem quality crystals are usually small and rarely exceed two grams, but can be faceted into attractive gems. Most of the facetable green, yellow and brown material have come from Franklin mining district, New Jersey, USA although blue one have come from Mt Saint Hilaire, Quebec, Canada. The sample (figure 1) considered in this work comes from Franklin.
In 1962, a cut willemite was reported by R. Crowningshield in the Fall edition of the Gems & Gemology journal[1].
 Figure 1. The 0.42 ct very-light-yellow willemite from Franlin mining
Figure 1. The 0.42 ct very-light-yellow willemite from Franlin miningdistrict, New Jersey, USA hosts numerous tiny inclusions.
| Shape | brilliant round |
| Size | Ø 4.4 x 2.6 mm |
| Color | very light yellow to slightly brown |
| Lustre | vitreous |
| Weight | 0.42 ct |
| SG | - |
| RI | 1.695 - 1.723 |
| DR | 0.028 U+ |
| Pleochroism | nil |
| Polariscope / Conoscope | anisotropic: light/dark 4 times / 360°, conoscope not used |
| SWUV | strong yellowish-green (see figure 2) |
| LWUV | inert |
| Magnetic susceptibility N52 | inert |
| Chelsea filter |
inert |
Table 1. Observational and measured properties
 Figure 2. The 0.42 ct very-light-yellow willemite seen under the
Figure 2. The 0.42 ct very-light-yellow willemite seen under theSWUV lamp exhibits a 'glowing' yellowish-green luminescence.
Infrared reflectance spectroscopy:
The IR reflectance spectrum (figure 3) was collected from the stone's table, the overall pattern indicates we are dealing with a sillicate. Unfortunately spectra references are missed but similarities can be established with the ATR spectrum of the R050652 willemite sample from Franklin available in RRUFF database[2]. There is no room left for doubt, the material is willemite. The spectrum can also be compared to the spectra of two willemites: Sio4 p377 and Sio23 p386[3], both from Franklin mining district, NJ, USA. Even if these spectra are acquired in IR transmission mode and are not directly comparable to reflectance ones because the relation between transmittance and reflectance is rather complex, one can state the material of this work is willemite.
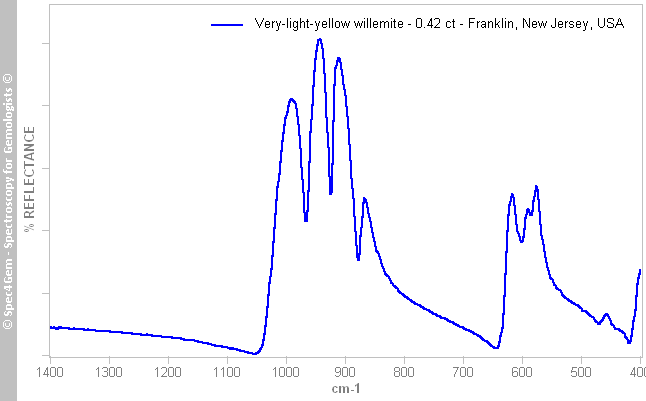 Figure 2. The IR reflectance spectrum of the 0.42 ct very-light-yellow willemite from Franklin mining district, New Jersey, USA, shows a sillicate pattern.
Figure 2. The IR reflectance spectrum of the 0.42 ct very-light-yellow willemite from Franklin mining district, New Jersey, USA, shows a sillicate pattern.UV-VIS-NIR spectroscopy:
The polarized UV-Vis-NIR spectra were collected although the pleochroism has not been observed (either too weak or unexistent). The spectra (figure 4) show low aborbance and details are almost hidden by the strong absorption from the blue to ultra-violet. The 450-700 nm range of the spectra is scaled to highlight the weak features. The polarization has few impact on the spectra, they are very similar and consist in an absorption continuum from the NIR to 450 nm followed by a stong absorption towards the UV. There are weak features at 458, 478, 488, 543, 585 and 650 nm, the 478, 488, 585 and 650 nm depend on the polarization.
 Figure 3. The polarized spectra of this willemite from Franklin are shown as is and scaled on the 450-700 nm spectral range to lay emphasis on the weak features. The spectra show Mn2+ features even if the main ones in the 400-500 nm spectral range are not resolved (see figure 5 for reference).
Figure 3. The polarized spectra of this willemite from Franklin are shown as is and scaled on the 450-700 nm spectral range to lay emphasis on the weak features. The spectra show Mn2+ features even if the main ones in the 400-500 nm spectral range are not resolved (see figure 5 for reference).The spectra are similar to the spectra (figure 5) of a bright-yellow willemite from Franklin (GRR1842 sample) published by Caltech[4] except for the 380-450 nm spectral range. The Caltech's sample shows strong bands in this spectral region range with significant bands at 423, 434 and 442 nm and a transmission window around 400 nm which do not exist as is in the willemite spectra of this work. Could these discrepencies be caused by the absorption of other impurities such as iron (Fe2+) presents in the Franklin deposits or by any other unexplained factors? The color cause of the Caltech's sample is Mn2+ in a tetrahedral site.
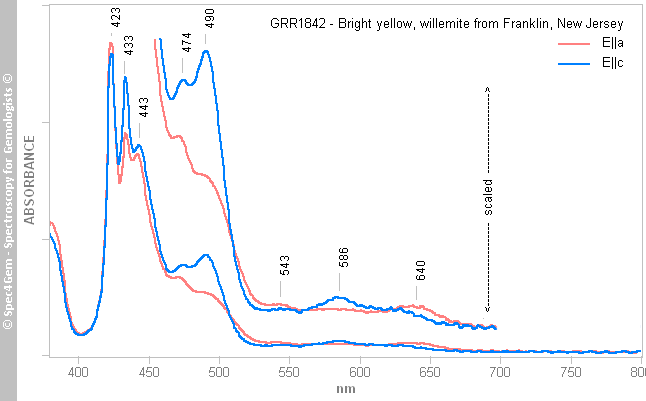 Figure 4. The polarized UV-Vis-NIR spectra of the Caltech's GRR1842 sample of a willemite from Franklin show Mn2+ features when occupying tetraedral sites. The 450-700 nm spectral range is scaled to lay emphasis on weak features.
Figure 4. The polarized UV-Vis-NIR spectra of the Caltech's GRR1842 sample of a willemite from Franklin show Mn2+ features when occupying tetraedral sites. The 450-700 nm spectral range is scaled to lay emphasis on weak features.Photoluminescence spectroscopy:
The photoluminescence spectroscopy was performed with four excitation sources: 254, 277, 405 and 442 nm. All sources produce the same emission spectrum with the sole difference, the emission level. Of course, all sources do not have the same power but even if the 405 nm laser power is relatively high, the emission level is very low compared to the emission produced by the 254 nm source. By contrast with the 405 nm source, the 442 nm source produces a quite high emission level. This is in line with the Mn2+ 400-450 nm absorptions observed in Caltech sample. To keep it simple, where the Mn2+ absorption does not exist, for instance at 405 nm, the Mn2+ emission is low although where it does exist, for instance at 442 nm, the emission is strong, this is connected to the excitation-emission principle. The figure 6 depicts the spectrum obtained with the 277 nm source, scaling the other spectra to adjust the maximum emission level produces fully identical spectra, for clarity only one was depicted.
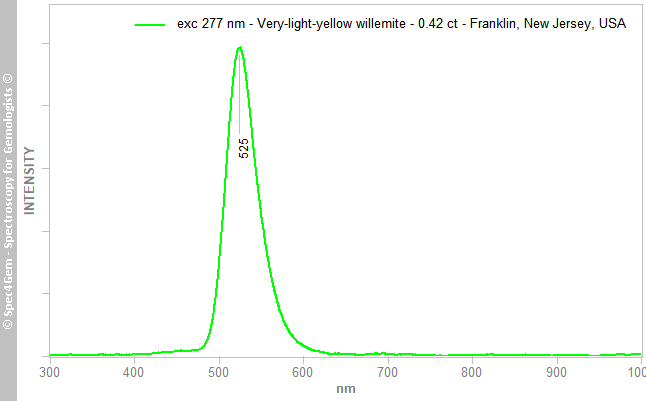 Figure 5 The photoluminescence spectrum of the Franlin willemite shows a yellowish-green emission centered at 525 nm while excited by the 254, 277, 405 and 442 nm sources, emissions only differ in intensity, the 405 nm source producing the weakest.
Figure 5 The photoluminescence spectrum of the Franlin willemite shows a yellowish-green emission centered at 525 nm while excited by the 254, 277, 405 and 442 nm sources, emissions only differ in intensity, the 405 nm source producing the weakest.The 525 nm emission center is well-known and is ascribed to Mn2+ replacing Zn2+, see Gaft et al[5], Gorobets and Rogojine[6]. The heavily-doped Zn2SiO4:Mn2+ phosphor was utilized within the white-light-emitting diode as a blue-to-green color conversion phosphor, therefore its luminescence has been thoroughly studied.
Conclusion:
The gemstone of this work coming from Franklin mining district, New Jersey, USA is a willemite as confirmed by the IR reflectance spectroscopy. Its very-light-yellow to brownish color is caused by the weak absorption continuum towards the UV 'ending' by an absorption edge at 450 nm that cannot be resolved as Mn2+ triplet (423, 433 and 443 nm). Mn2+ does not have any absorption around 400 nm but this is not observed in this willemite. From both factors, it is impossible to definitely state on the fact the gemstone is only colored by Mn2+. On the flip side, photoluminescence spectroscospy demonstrates that Mn2+ emission is strong for 254, 277 and 442 nm excitations and weak for 405 nm which are in line with Mn2+ absorptions. Even if there is no room left for the presence of Mn2+ substituing Zn2+ in this willemite, it is not proven it is the only color cause.
[1] Developments and highlights at the Gem Trade Lab in N.Y. - Willemite, R. Crowningshield, Gems & Gemology, Fall 1962, Vol. 10, pp. 338-339
[2] RRUFF database - Willemite R050652
[3] Infrared spectra of mineral species, Nikita V. Chukanov, 2014, Springer Editor, ISBN: 978-94-007-7128-4
[4] Willemite Visible Spectra - List of Visible Data Files on the Caltech Mineral Spectroscopy Server
[5] Modern Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, 2015, Springer Editor, 2nd Edition, ISBN: 9783319247632
[6] Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002, ISBN: 5901837053, p. 176

