CGS:Cr4+, a greenish-blue synthetic melilite
- Details
- Created: Sunday, 22 April 2018 10:58
CGS stands for Calcium Gallium Silicate and it belongs to the melilite-type with its formula Ca2Ga2SiO7. This material is synthesized by Czochralski process and doped with chromium as Cr4+. This material was faceted (rare) to give the 4.15 ct gemstone shown in figure 1. The greenish-blue color is very attractive and the high lustre (sub-adamantine) makes the stone an interesting one. To the unaided eye, the stone evenly looks greenish-blue but the picture reveals a bluish-green tint. The material is strongly pleochroic (bluish-green / very slightly greenish-blue) and the cut was oriented to minimize the bluish-green. Through the pavilion, a curved blue color zonation can be observed but it does not impair the beauty of the stone. Hardness is unknown.
 Figure 1. 4.15 ct greenish-blue CGS:Cr4+ (synthetic melilite)
Figure 1. 4.15 ct greenish-blue CGS:Cr4+ (synthetic melilite)| Shape | a kind of cushion |
| Size | 8.8 x 8.9 x 4.8 mm |
| Color | greenish-blue |
| Lustre | sub-adamantine |
| Weight | 4.15 ct |
| SG | 4.69 [4.07 [1]] |
| RI | OTL, measured by reflectivity: ~1.82/1.84 [ ? ] |
| DR | facet edges are doubled, estimated DR should be around 0.010 - 0.015 |
| Pleochroism | strong: bluish-green / very slightly greenish-blue |
| Polariscope / Conoscope | anisotropic, uniaxial |
| SWUV | inert |
| LWUV | inert |
| Magnetic susceptibility N52 | diamagnetic |
| Chelsea filter | 'inert' |
Table 1. Observational and measured properties
Infrared reflectance spectroscopy:
The IR reflectance spectrum (figure 2) was acquired from the stone's table. It shows a simple silicate spectrum with its bands over the 900-1100 cm-1 range. Unfortunately, there is no melilite-type material in the library for comparison.
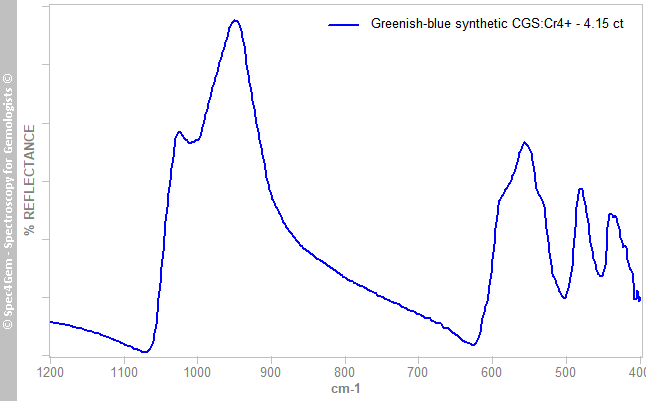 Figure 2. The IR reflectance spectrum of this 4.15 ct greenish-blue CGS:Cr4+ shows a silicate pattern with its bands over the 900-1100 cm-1 range.
Figure 2. The IR reflectance spectrum of this 4.15 ct greenish-blue CGS:Cr4+ shows a silicate pattern with its bands over the 900-1100 cm-1 range.UV-VIS-NIR spectroscopy:
The UV-Vis-NIR spectra (figure 3) were acquired with the light path traveling from girdle to opposite girdle and perpendicular to the optic axis. Collecting through the stone's table does not enable to split the E-ray from the O-ray even with polarizing filters.
The spectra clearly show the difference between both rays and thus the difference in color (pleochroism), there is more green and yellow in the O-ray. The bands between 500 and 800 nm result from the Cr4+ spin-forbidden transitions.
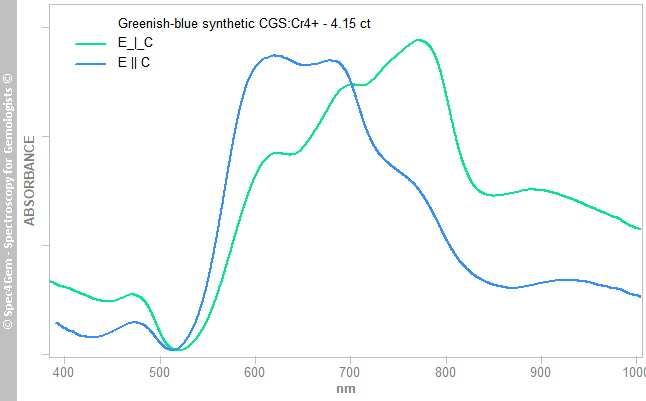 Figure 3. Polarized UV-Vis-NIR spectra of the 4.15 ct grennish-blue CGS:Cr4+. The bands between 500 and 800 nm result from the Cr4+ spin-forbidden transitions.
Figure 3. Polarized UV-Vis-NIR spectra of the 4.15 ct grennish-blue CGS:Cr4+. The bands between 500 and 800 nm result from the Cr4+ spin-forbidden transitions. Photoluminescence spectroscopy:
No photoluminescence with the following sources: 254, 377, 405, 532 and 670 nm.
Conclusion:
This CGS material is likely intended for optics but with Cr4+ doping it has a very attractive color. Anyway, it makes a good sample for studying the Cr4+ absorption in solids.

