Pink fluorite from Mont Blanc massif, France
- Details
- Created: Sunday, 19 March 2017 10:58
Pink fluorite is found in Mont Blanc massif, either in France or in Switzerland, the 2.60 ct light-pink fluorite gemstone shown in figure 1 is a sample that reportedly comes from the french border.
The pink color is the result of:
- fluorine rose (Mont Blanc) - "centre complexe YO2 (ion yttrium trivalent plus ion moléculaire d'oxygène en tri négation)" - Geowiki[1],
- fluorite, pink, Huanzala, Peru - "the 485 nm band from a O23- molecular ion stabilized by an adjacent Y3+ ion in addition to the 400 and 580 nm bands from the Y associated F-center" - Caltech[2].
 Figure 1. 2.60 ct light pink fluorite from Mont Blanc massif, France.
Figure 1. 2.60 ct light pink fluorite from Mont Blanc massif, France.| Shape | round |
| Size | Ø 9.0 x 5.0 mm |
| Color | light pink |
| Lustre | sub vitreous |
| Weight | 2.60 |
| SG | 3.17 |
| RI | 1.436 |
| DR | - |
| Pleochroism | - |
| Polariscope / Conoscope | Isotropic |
| SWUV | inert |
| LWUV | inert |
| Magnetic susceptibility |
Table 1. Observational and measured properties
Infrared reflectance spectroscopy:
The IR reflectance spectrum (figure 2) of this 2.60 ct light pink fluorite gemstone from Mont Blanc massif in France was acquired from its table. There is no particular information, the spectrum is a characteristic fluorite IR reflectance spectrum.
 Figure 2. IR reflectance spectrum of the 2.60 ct light pink fluorite from Mont Blanc massif, France.
Figure 2. IR reflectance spectrum of the 2.60 ct light pink fluorite from Mont Blanc massif, France. UV-VIS-NIR spectroscopy:
The UV-Vis-NIR absorption spectrum was acquired with a light path perpendicular to the table, the light beam entering the stone through the culet. The light path within the stone is about 5 mm.
The color of the gemstone is light to very light, the absorption levels are very low unlike it may appear in figure 3. The full absorption scale is 0.1 (A) that corresponds to an absorption coefficient per centimetre (α) of only 0.2 / cm. If the full scale was set to 1, the spectrum would have been almost flat! Of course, the orange-red is more transmitted than the green and slightly more than violet-blue, but the difference of transmission is low, that explaining the pink color.
 Figure 3. UV-Vis absorption spectrum of the 2.60 ct light pink fluorite from Mont Blanc massif, France. The absorbance full scale is only 0.1 (A) which corresponds to a absorption coefficient (α) of almost 0.2 (A.cm-1). The main absorption band centered at 490nm absorbs the violet, blue, green and yellow. An additional well formed absorption band is present in the UV at 366 nm. There is a possible band at 406 nm but as it is so weak, it is unreasonable to consider it.
Figure 3. UV-Vis absorption spectrum of the 2.60 ct light pink fluorite from Mont Blanc massif, France. The absorbance full scale is only 0.1 (A) which corresponds to a absorption coefficient (α) of almost 0.2 (A.cm-1). The main absorption band centered at 490nm absorbs the violet, blue, green and yellow. An additional well formed absorption band is present in the UV at 366 nm. There is a possible band at 406 nm but as it is so weak, it is unreasonable to consider it. According to Caltech's data[2], the band at 490 nm is ascribed to a "O23- molecular ion stabilized by an adjacent Y3+ (yttrium) ion". Unlike the published spectrum (figure 6) of the pink fluorite from Huanzala, Peru, the 400 and 580 nm strong bands attributed to F-centers associated with Y3+ are not directly observed, or perhaps except for the 400 nm band that might be present in the spectrum at 406 nm. Gaussian fitting simulations were experimented (figure 4 and figure 5), each gaussian is in red and the sum of all the gaussians is in yellow. The simplest simulation (figure 4) using only four gaussians at 360, 365, 407 and 487 nm produces an almost perfect fit. This simulation does not include any 580-590 band for Y3+ associated to the F-centers.
 Figure 4. UV-Vis absorption spectrum gaussian fitting simulation with only four bands at 360, 365, 407 and 487 nm produces an almost perfect fit. This simulation does not include any 580-590 band for Y3+ associated to the F-centers.
Figure 4. UV-Vis absorption spectrum gaussian fitting simulation with only four bands at 360, 365, 407 and 487 nm produces an almost perfect fit. This simulation does not include any 580-590 band for Y3+ associated to the F-centers. Another gaussian fitting simulation (figure 5) was experimented with an additional band to the existing simulation of figure 4. The 595 band was added to check whether the Y3+ band could exist in the spectrum of this 2.60 ct pink fluorite. Here again the fitting is almost perfect and the bands positions are 361, 365, 404, 487 and 595 nm. Even if the 404 and 595 nm band could exist, they poorly contribute to the spectrum compared to the O23- band at 487 nm.
 Figure 5. UV-Vis absorption spectrum gaussian fitting simulation with five bands at 361, 365, 404, 487 and 595 nm produces an almost perfect fit. Unlike the simulation of figure 4, this simulation includes a 595 nm band for Y3+ associated to the F-centers. Assuming the 404 and 595 nm bands do exist in the spectrum of this fluorite, their contribution to the spectrum is very poor compared to that of O23- at 487 nm.
Figure 5. UV-Vis absorption spectrum gaussian fitting simulation with five bands at 361, 365, 404, 487 and 595 nm produces an almost perfect fit. Unlike the simulation of figure 4, this simulation includes a 595 nm band for Y3+ associated to the F-centers. Assuming the 404 and 595 nm bands do exist in the spectrum of this fluorite, their contribution to the spectrum is very poor compared to that of O23- at 487 nm. 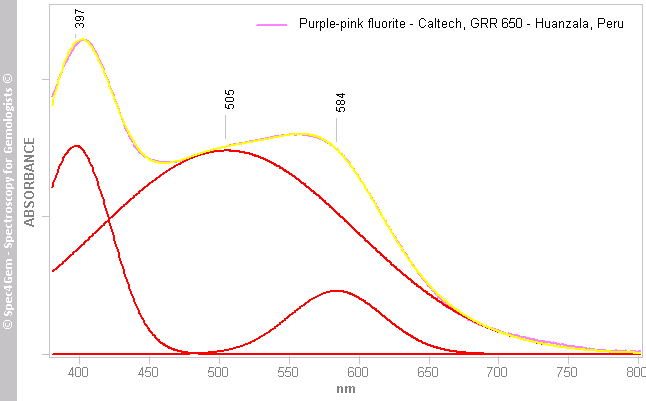 Figure 6. UV-Vis absorption spectrum of the Caltech's pink fluorite from Huanzala, Peru (GRR650) with its gaussian fitting simulation involving three bands at 397, 505 and 584 nm. The 397 and 584 nm bands are attributed to the F-centers associated to Y3+ ions and the 505 nm band is ascribed to a O23- molecular ion stabilized by an adjacent Y3+.
Figure 6. UV-Vis absorption spectrum of the Caltech's pink fluorite from Huanzala, Peru (GRR650) with its gaussian fitting simulation involving three bands at 397, 505 and 584 nm. The 397 and 584 nm bands are attributed to the F-centers associated to Y3+ ions and the 505 nm band is ascribed to a O23- molecular ion stabilized by an adjacent Y3+.Photoluminescence spectroscopy:
Sources such as 254, 280, 370, 405, 532, 650 and 780 nm do not induce any observable photoluminescence.
Conclusion:
This pink fluorite from Mont Blanc massif in France has common gemological properties for fluorite specie. It does not any photoluminescence even with the power of the exciting lasers. The pink color results from a good transmission ( >80%) over the all UV-Vis spectral range with the orange and red slightly privileged, as well as the violet-blue but to a lesser extent.
The color cause can reliably attributed to O23- molecular ion stabilized by adjacent Y3+ ions without the contribution (or a poor contribution) of the F-centers associated with Y3+ as shown by the UV-Vis spectrum and the band fitting simulations.

