Rare 1.57 ct purple hydroxyherderite from Virgem da Lapa, Brazil
- Details
- Created: Thursday, 14 November 2019 10:58
Initially published as a Gem note in the Journal Of Gemmology of the Gem-A. 2019, 36(6), 501–503.
http://doi.org/10.15506/JoG.2019.36.6.501
Hydroxylherderite [CaBePO4(OH,F)] is a phosphate mineral that forms a solid-solution series with herderite in which hydroxyl (OH) is dominant over fluorine (F). The term herderite is commonly used to refer to both minerals of the herderite-hydroxylherderite series, although most specimens on the market are actually hydroxylherderite (www.mindat.org/min-1876.html). Mainly found in granitic pegmatites, herderite can crystallise in attractive well-formed crystals and is sometimes transparent enough for faceting. Most gem-quality herderite comes from Minas Gerais in Brazil (Johnson 1996[1]; Wentzell 2004[2]) and from northern Pakistan (Laurs & Quinn 2006[3]). The mineral series comes in a limited range of colors, including colorless, grey, brown, pale yellow, pale green and greenish yellow, and much more rarely light blue and purple.
The 1.57 ct round brilliant-cut gemstone described in this report shows an unusually strong purple color (Figure 1), and was therefore characterised in detail. It reportedly came from the Virgem da Lapa in the Araçuaí region of Minas Gerais, Brazil. One deposit in this area, the Xanda (Xandra) mine, is a known source of hydroxylherderite — especially showing purple color — along with other minerals such as feldspar, tourmaline, muscovite and quartz (Dunn et al. 1979[4]; www.mindat.org/loc-6818.html).
 Figure 1. The faceted purple hydroxylherderite from Virgem da Lapa
Figure 1. The faceted purple hydroxylherderite from Virgem da Lapadescribed here weighs 1.57 ct.
Photo by T. Cathelineau.
| Shape | round, 'flat' brilliant cut |
| Size | Ø 7.5 x 4.5 mm |
| Color | slightly greyish purple overall, appearing greyish blue in daylight (and under ‘cool white’ bulbs) or slightly greyish purple to purple in incandescent light |
| Lustre | sub-vitreous |
| Weight | 1.57 ct |
| SG | 3.02 [2.95 – 3.02][5] |
| RI | 1.590 - 1.620 [1.556 – 1.620][5] |
| DR | 0.030 biaxial negative [0.029 – 0.030, B-][5] |
| Pleochroism | strong, in purple and almost colorless (a third color was not observed, although herderite is biaxial) |
| Polariscope / Conoscope | not tested |
| SWUV | inert |
| LWUV | inert |
| Magnetic susceptibility N52 | inert |
| Chelsea filter | no reaction |
Table 1. Observational and measured properties
Microscopic examination revealed moderate doubling and a few fissures and partially healed fractures. The RI of herderite varies with the F/OH ratio (Leavens et al. 1978[6]), and the measured RIs of 1.590–1.620 correspond to a herderite (F) content of 43% ± 1% and thus a hydroxylherderite (OH) content of 57% ± 1%. Since the sample’s composition falls on the hydroxyl-rich side of the series, it is hydroxylherderite.
Infrared reflectance spectroscopy:
The infrared reflectance spectrum (Figure 2) was collected from the stone’s table facet without regard for polarisation or orientation. The spectrum covered the 2000-400 cm–1 range, although Figure 2 is truncated above 1400 cm–1 because no features were recorded in that range. Due to the setup of the FTIR spectrometer, the water and hydroxyl region around 3600 cm–1 was not measured. The major bands in the ranges 1200-1000 cm–1 and 600-500 cm–1 are characteristic of the PO43– ion, and confirm the presence of a phosphate mineral. Comparison of the spectrum to the herderite-hydroxylherderite series in the RRUFF database — although the RRUFF spectra were not collected in reflectance mode, but rather using the ATR method — and to the IR transmission spectra published by Chukanov (2014)[7], unambiguously identified the sample as herderite-hydroxylherderite. However, without information on the bands in the 3600 cm–1 region it is impossible to conclude whether a sample is herderite or hydroxylherderite (Frost et al. 2014[8]).
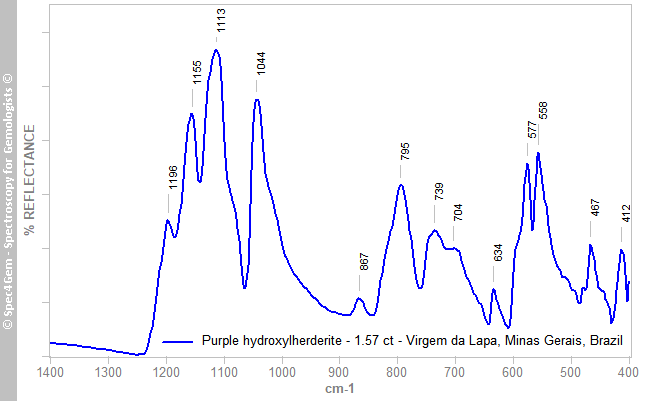 Figure 2. The IR reflectance spectrum characterizes the stone as a member of the herderite-hydroxylherderite series.
Figure 2. The IR reflectance spectrum characterizes the stone as a member of the herderite-hydroxylherderite series.UV-VIS-NIR spectroscopy:
The herderite-hydroxylherderite series is optically biaxial so, as mentioned above, one might expect three colors associated with the three polarization directions. Collecting the Vis-NIR spectrum for each of the three directions is typically not feasible for a faceted gemstone, so spectra were recorded for only two of them using a polarizing filter with the beam oriented parallel to the direction of maximum pleochroism. The resulting two spectra (Figure 3) correspond to the observed pleochroic colors of purple and almost colorless. The spectrum associated with the purple color showed a strong absorption edge in the violet region and a strong and broad absorption band at ~577 nm (yellow region), creating two transmission windows at ~470 nm (blue region) and above ~660 nm (red and NIR region). Also present were a weak but broad band centred at ~740 nm and a doublet at 969/981 nm in the NIR region (the latter being linked to the second overtone [3νOH] of the OH fundamental absorptions in the 3600 cm–1 region; Frost et al. 2014[8]). The spectrum associated with the almost colorless direction had similar — but much weaker — features, as well as a shoulder at 422 nm. The stone’s purple color is related to the transmission windows in the blue and red regions of the spectrum. Such coupled transmission windows are often responsible for color-change behaviour, as was also seen in this hydroxylherderite.
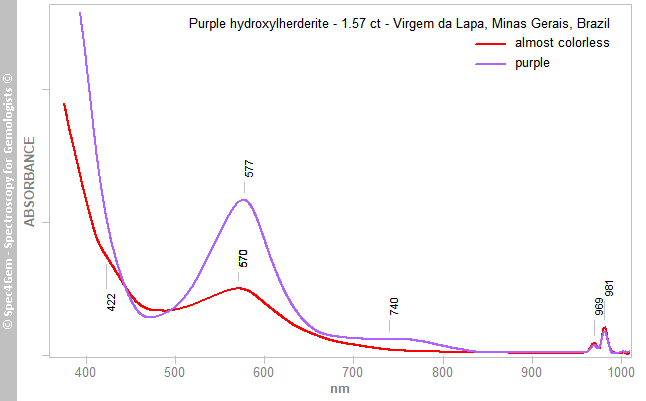 Figure 3. Vis-NIR spectra were collected for the purple and almost colorless pleochroic directions of the hydroxylherderite. Transmission windows in the blue (~470 nm) and red (~660–720 nm) regions are responsible for the purple color of the stone, as well as its color change.
Figure 3. Vis-NIR spectra were collected for the purple and almost colorless pleochroic directions of the hydroxylherderite. Transmission windows in the blue (~470 nm) and red (~660–720 nm) regions are responsible for the purple color of the stone, as well as its color change.Photoluminescence spectroscopy:
The stone’s photoluminescence (PL) was studied using four excitation sources: 254, 280, 377 and 405 nm, but only the last gave any results. The 405 nm laser produced a main large emission peak centred at 573 nm and a weaker emission at 448 nm (Figure 4). These features are ascribed to Mn2+ and Eu2+, respectively (Gorobets & Rogojine 2001[9])
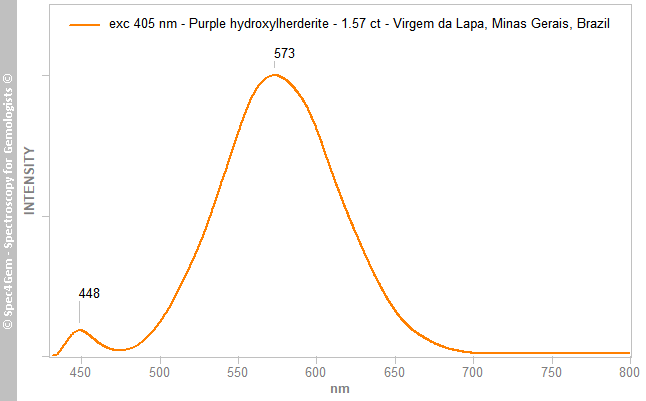 Figure 4. The PL spectrum of the purple hydroxylherderite obtained with 405 nm excitation shows a strong emission at 573 nm that is indicative of Mn2+.
Figure 4. The PL spectrum of the purple hydroxylherderite obtained with 405 nm excitation shows a strong emission at 573 nm that is indicative of Mn2+.Conclusion:
While it is tempting to ascribe the cause of the purple coloration to Mn, further investigations are required. The spectral pattern across the visible range shows similarities to those of pink and purple apatite (color caused by F centers), deep violet apatite (Mn5+), blue and violet fluorite (F centers), and even amethyst (radiation-induced color centers).
[1] Gem Trade Lab Notes: Herderite. Johnson, M.L. 1996. Gems & Gemology, 32(3), 208
[2] Lab Notes: Herderite update. Wentzell, C.Y. 2004. Gems & Gemology, 40(1), 61–62
[3] Gem News International: Herderite from Pakistan. Laurs, B.M. & Quinn, E.P. 2006. Gems & Gemology, 42(2), 174–175
[4] Hydroxyl-herderite from Brazil, and a guide to species nomenclature for the herderite/hydroxyl-herderite series. Dunn, P.J., Wolfe, C.W., Leavens, P.B. & Wilson, W.E. 1979. Mineralogical Record, 10(1), 5–11
[5] Gems. O’Donoghue, M. (ed) 2006. Butterworth-Heinemann, Oxford, 873 pp [see p 416]
[6] Compositional and refractive index variations of the herderite-hydroxyl-herderite series. Leavens, P.B., Dunn, P.J. & Gaines, R.V. 1978. American Mineralogist, 63(9–10), 913–917
[7] Infrared Spectra of Mineral Species. Chukanov, N.V. 2014. Springer, Dordrecht, The Netherlands, 1,726 pp [see pp 1167–1168, 1194 and 1318], http://doi.org/10.1007/978-94-007-7128-4
[8] Raman, infrared and near-infrared spectroscopic characterization of the herderite–hydroxylherderite mineral series. Frost, R.L., Scholz, R., López, A., Xi, Y., Queiroz, C. de S., Belotti, F.M. & Filho, M.C. 2014. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 118, 430–437, http://doi.org/10.1016/j.saa.2013.09.021.
[9] Luminescent Spectra of Minerals. Gorobets, B.S. & Rogojine, A.A. 2001. RCP VIMS, Moscow, Russia, 215 pp [see p 135]

