Aesthetic Mesolite Cabochons from India
- Details
- Created: Monday, 06 January 2020 20:48
Initially published as a Gem note in the Journal Of Gemmology of the Gem-A. 2019, 36(7), 585–587.
http://doi.org/10.15506/JoG.2019.36.7.585
Well-known amongst mineral collectors, zeolite minerals are sometimes encountered as collector gemstones (e.g. pollucite, leucite, natrolite, analcime, etc.). Gem- and specimen-grade zeolites commonly form as secondary minerals within cavities hosted by basalts, and perhaps the most famous deposits are hosted by the Deccan Volcanic Province in India (Ottens et al. 2019)[1]. During the past few years, white cabochons composed of radiating crystal clusters described as scolecite (a zeolite of the natrolite subgroup) have been available from India. Early in 2019, similar stones with mixed white and orangey pink (‘salmon’) colouration exhibiting aesthetic patterns (e.g. Figure 1) entered the market from India’s Maharashtra State. Two such samples (28.10 and 36.73 ct) were characterized for this report (Figure 1).
Natrolite-subgroup zeolites are composed of three main species: scolecite (Ca), natrolite (Na) and mesolite (Ca and Na). They commonly form attractive sprays of slender radiating crystals up to 30 cm in size, in colourless, white, grey, brown, bluish, yellowish, or more rarely pink or orange. The orthorhombic natrolite and the monoclinic mesolite and scolecite all form pseudo-tetragonal prisms with an almost square section. If a cabochon of such aggregates is cut perpendicular to the prisms, a chequered pattern may result as shown by the 28.10 ct stone in Figure 1. When cut parallel to the slender crystals, they produce a radiating acicular pattern, as shown by the 36.73 ct cabochon in Figure 1.
 Figure 1. The cabochons from Maharashtra State in India
Figure 1. The cabochons from Maharashtra State in Indiaexamined for this study (28.70 ct, bottom, and 36.73 ct, top) show
aesthetic patterns due to differently coloured mesolite aggregates.
Photo by T. Cathelineau.
| Shape | pear |
| Size | 34.3 x 26.7 x 7.2 mm |
| Color | 'salmon' and white |
| Lustre / Diaphaneity | dull / translucent in the ‘salmon’ areas and opaque in the white ones |
| Weight | 36.73 ct, increased in weight when left immersed in water for several minutes |
| SG | 2.23 [2.27][2] |
| RI | 1.505 [1.5048 - 1.5053][2] |
| DR | none, could not be measured [0.001 B+][2] |
| Pleochroism | - |
| Polariscope / Conoscope | - |
| Spectrocope | strong absorption in the violet and blue regions with moderate absorption in the green |
| SWUV | faint white in white areas and inert in ‘salmon’ portions |
| LWUV | inert |
| Magnetic susceptibility N52 | diamagnetic |
| Chelsea filter | no reaction |
Table 1. Observational and measured properties
The 28.10 ct stone showed the same properties except that it was opaque and had an SG value of 2.18; no RI or spectrum could be obtained. (Although mesolite is biaxial positive, all three RI values are ~1.505, so birefringence is almost unobservable with gemmological refractometer.)
Infrared reflectance spectroscopy:
Infrared reflectance spectra were collected from several spots on the cabochons (both ‘salmon’ and white areas), and all yielded the same pattern (e.g. Figure 2). The spectra are characteristic of a silicate mineral, and comparison with the zeolites database revealed an exact match with mesolite samples analysed a few years ago. The identification of earlier samples as mesolite was achieved by comparing their IR spectra to those of the natrolite subgroup in the RRUFF database and to the IR transmission spectra published by Chukanov (2014, pp 846, 848, 869)[3]. The 1655 and 1638 cm–1 bands are associated with H2O bending vibrations (Geiger 2012)[4].
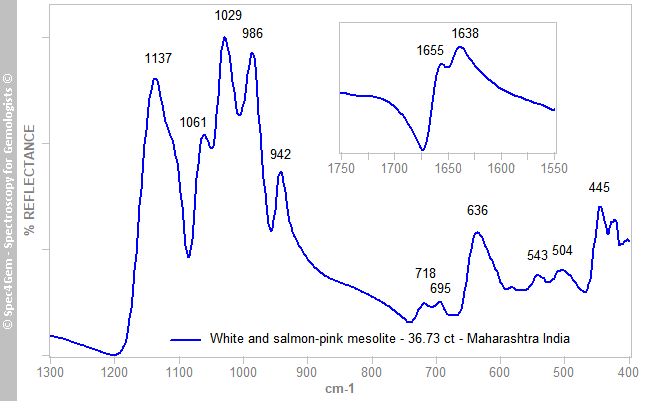 Figure 2. The IR reflectance spectrum characterizes the stones in Figure 1 as mesolite, the Ca-Na member of the natrolite subgroup. The 1655 and 1638 cm–1 bands (inset) indicate H2O bending vibrations.
Figure 2. The IR reflectance spectrum characterizes the stones in Figure 1 as mesolite, the Ca-Na member of the natrolite subgroup. The 1655 and 1638 cm–1 bands (inset) indicate H2O bending vibrations.UV-VIS-NIR spectroscopy:
A Vis-NIR spectrum (Figure 3) was collected from a translucent ‘salmon’ area of the 36.73 ct cabochon with the beam oriented nearly perpendicular to the mesolite prisms. It showed strong absorption in the violet, blue and green regions with an edge in the yellow-green region caused by a prominent band at ~535 nm. Also present was a moderate band at ~665 nm (red region), a weak band at ~855 nm and a moderate band at ~980 nm (NIR). The overall spectral pattern displayed an absorption continuum towards the UV and is responsible for the ‘salmon’ colour. Iron is easily incorporated into zeolites as demonstrated by Suhartana et al. (2018)[5]; Fe-bearing zeolite is generally orange-brown, which is consistent with the samples’ colouration although the author is not aware of any equivalent Vis-NIR spectra. The 980 nm band is very likely due to OH second overtones, as deduced by the author from the OH stretching vibrations of H2O between 3600 and 3100 cm−1 (Geiger 2012)[4].
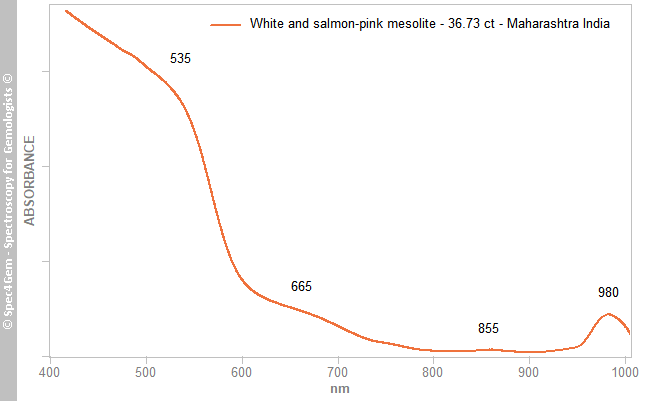 Figure 3. This Vis-NIR spectrum was collected from a ‘salmon’ area of the 36.73 ct mesolite. A continuum of absorption in the violet, blue and, to a lesser extent, green regions gives the stone its ‘salmon’ colour. The path length of the beam was ~6 mm.
Figure 3. This Vis-NIR spectrum was collected from a ‘salmon’ area of the 36.73 ct mesolite. A continuum of absorption in the violet, blue and, to a lesser extent, green regions gives the stone its ‘salmon’ colour. The path length of the beam was ~6 mm.Photoluminescence spectroscopy:
Photoluminescence of the white areas of the cabochons was studied using 254 nm excitation, in accordance with the fluorescence observations mentioned above. (Other wavelengths at 377, 405 and 447 nm did not produce any photoluminescence.) The 254 nm source induced a broad but very weak emission peaking at 480 nm (Figure 4). This spectrum is similar to those of zeolites published without explanation by Gorobets & Rogojine (2002, p 150)[6]. It is possible that the luminescence could be related to organic molecules and/or an oxygen centre.
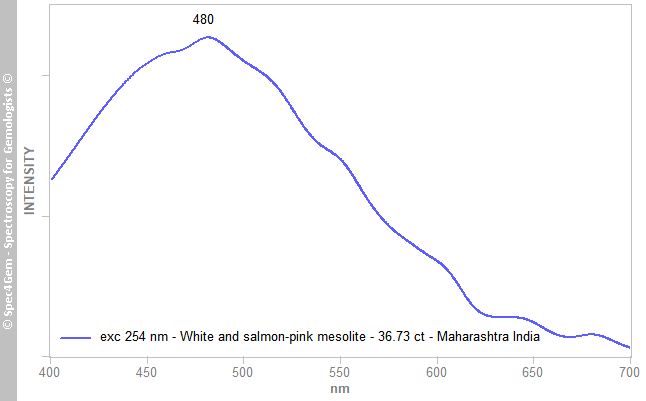 Figure 4. The photoluminescence spectrum of a white area of the 36.73 ct mesolite obtained with 254 nm excitation shows a broad but weak emission centred at 480 nm.
Figure 4. The photoluminescence spectrum of a white area of the 36.73 ct mesolite obtained with 254 nm excitation shows a broad but weak emission centred at 480 nm.[1] Exceptional multi stage mineralization of secondary minerals in cavities of flood basalts from the Deccan Volcanic Province, India. Ottens, B., Götze, J., Schuster, R., Krenn, K., Hauzenberger, C., Zsolt, B. & Vennemann, T. 2019. Minerals, 9(6), article 351 (41 pp), http://doi.org/10.3390/min9060351.
[2] Gems. O’Donoghue, M. (ed) 2006. Butterworth-Heinemann, Oxford, 873 pp [see p 428]
[3] Infrared Spectra of Mineral Species. Chukanov, N.V. 2014. Springer, Dordrecht, The Netherlands, 1,726 pp [see pp 846, 848 and 869], http://doi.org/10.1007/978-94-007-7128-4
[4] A low-temperature IR spectroscopic investigation of the H2O molecules in the zeolite mesolite. Geiger, C.A. 2012. European Journal of Mineralogy, 24(3), 439–445, http://doi.org/10.1127/0935-1221/2012/0024-2176.
[5] Modification of natural zeolite with Fe(III) and its application as adsorbent chloride and carbonate ions. Suhartana, Sukmasari, E. & Azmiyawati, C. 2018. IOP Conference Series: Materials Science and Engineering, 349, article 012075 (12 pp), http://doi.org/10.1088/1757-899x/349/1/012075.
[6] Luminescent Spectra of Minerals. Gorobets, B.S. & Rogojine, A.A. 2002. RCP VIMS, Moscow, Russia, 300 pp [see p 150]

