3.17ct Vivid-green zircon from Sri Lanka
- Details
- Created: Friday, 08 February 2019 11:02
Green zircons are exceptionally rare especially in pleasant green colors. They form as the result of natural radiation damage in a process known as metamictization. Over long periods of geological time, radiation produces changes in the crystal lattice so that these metamict zircons are almost amorphous rather than crystalline. These rare specimens are coveted by collectors and most of time they should not be worn as jewellery since they are often rather highly radioactive.
If we consider this zircon with a radioactivity on average of 0.80 μSv.h-1, wearing it as a ring 24 hours a day during only 52 days makes your finger absorb 1 mSv that is the maximum acceptable yearly exposition in France. It is equivalent to wear it 3 to 4 hours a day during 365 days.
The vivid-green color makes it very attractive as shown in figure 1.
 Figure 1. Metamict 3.17 ct vivid-green zircon from Sri Lanka.
Figure 1. Metamict 3.17 ct vivid-green zircon from Sri Lanka.| Shape | rounded cushion, crown: cushion cut, pavillion: step-cut |
| Size | 8.8 x 7.2 x 5.6 mm |
| Color | vivid green to yellowish-green |
| Lustre | vitreous to sub-adamantine |
| Weight | 3.17 ct |
| SG | 3.91 |
| RI | 1.88-1.89 (mesured by reflectivity) |
| DR | doubling of facets' edges observed with the 10x lens |
| Pleochroism | green / slightly brownish green |
| Polariscope / Conoscope | anisotropic: light/dark 4 times / 360°, conoscope: uniaxial figure observed down to the stone's length |
| SWUV | inert |
| LWUV | inert |
| Magnetic susceptibility N52 | inert |
| Chelsea filter | reddish |
| Radioactivity |  0.70-0.90 μSv.h-1 (150-200 cpm) 0.70-0.90 μSv.h-1 (150-200 cpm) |
Table 1. Observational and measured properties
Infrared reflectance spectroscopy:
The infrared reflectance spectrum (figure 2) was collected from the gemstone's table without any particular orientation / polarization. Most of the bands are 'diffused' and the silicate bands in the 800-1200 cm-1 are very rounded. Such spectrum pattern indicates the zircon crystal lattice has partialy been destroyed by radiation according to metamictization process that transform well organized lattice into almost amorphous structure. This process is well known in Sri Lankan zircon because of their heigh Uranium content. This sample is not fully metamict since the bands are still visible and it is consistent with the RI and SG that indicate a low-zircon but still in the upper range.
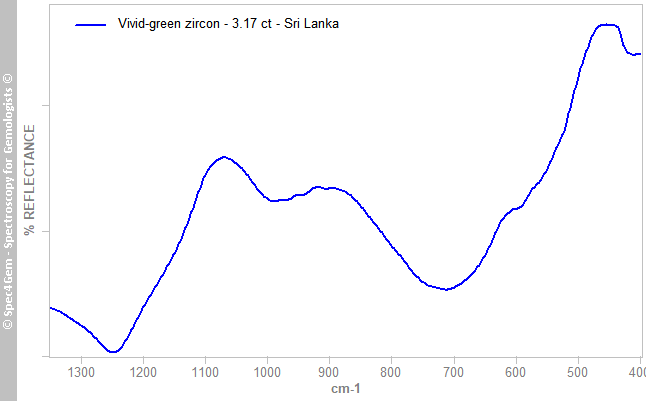 Figure 2. IR reflectance spectrum of the 3.17ct vivid-green zircon from Sri Lanka showing the result of metamictization on zircon material.
Figure 2. IR reflectance spectrum of the 3.17ct vivid-green zircon from Sri Lanka showing the result of metamictization on zircon material.UV-VIS-NIR spectroscopy:
The zircon being anisotropic and a weak to moderate pleochroism has been observed, polarized UV-Vis-NIR spectra were collected for the polarizations E || C and E_|_C and are depicted in figure 3 where E || C spectrum was shifted for clarity. The two spectra have a similar pattern with a strong absorption in the UV, violet and blue, a prominent absorption around 650-660 nm in the red. Multiple absorption bands are dispatched on all the spectrum range with slight differences in the positions between the two polarizations. Another interesting feature is the broadness of the bands in comparison to a high-zircon showing Uranium spectrum. The metamictization state of the gemstone is explaining such band broadness.
The transmission window in the green-yellow range with the maximum around 560-570 nm explains the vivid-green color. The complexity of spectrum is ascribed to U4+ [1].
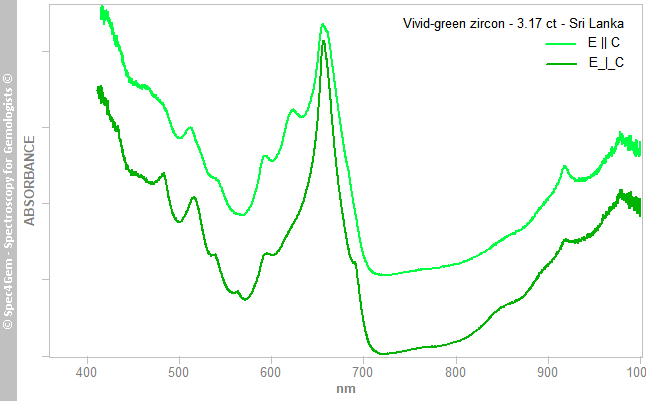 Figure 3. Polarized spectra (E||C is shifted for clarity) of the 3.17 ct vivid-green zircon from Sri Lanka showing the characteristic U4+ features with the band broadness due to metamictization.
Figure 3. Polarized spectra (E||C is shifted for clarity) of the 3.17 ct vivid-green zircon from Sri Lanka showing the characteristic U4+ features with the band broadness due to metamictization. Photoluminescence spectroscopy:
The luminescence of this zircon is almost nonexistent, 254 and 377 nm sources do not induce any luminescence and the 405 nm laser source produces a very weak luminescence that required at least 2 seconds integration time for a very weak signal. The collected spectrum is shown in figure 4. The weak emissions are cluttered by signal noise. The emission groups are possibly assigned to REE3+ [2][3], with Dy3+ at 478, 488 nm and 569-582 nm, Tb/Er3+ at 525-535 and 548-555 nm, Sm3+ at 604-620 and 649-670 nm. The emission groups pattern between 500 and 600 nm do not match the UO22+ luminescence pattern.
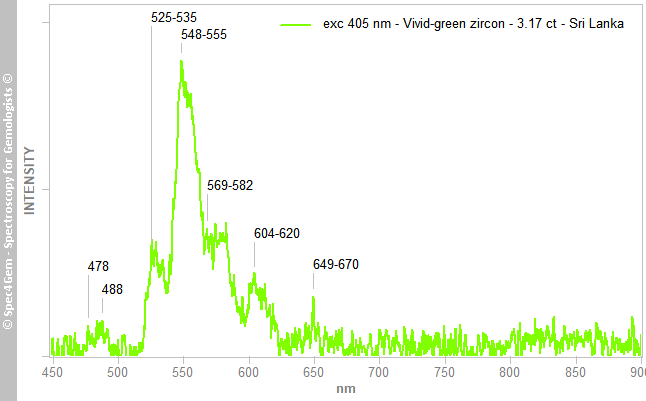 Figure 4. 405 nm photoluminescence spectrum of the 3.17 ct vivid-green zircon from Sri Lanka with very weak emissions likely connected to REE3+ such as Dy3+, Tb/Er3+ and Sm3+.
Figure 4. 405 nm photoluminescence spectrum of the 3.17 ct vivid-green zircon from Sri Lanka with very weak emissions likely connected to REE3+ such as Dy3+, Tb/Er3+ and Sm3+.Conclusion:
This vivid-green zircon from Sri Lanka is undergoing the metamictization process started a very long time ago and will infinitely continue, at least at human time scale. The metamictization results are observed in IR and UV-Vis spectra, the band broadness is very significant. The green color of the stone is due to U4+ which is present in quite high concentration (radioactivity level) and the band broadness as well. The photoluminescence just revealed some REE3+ traces that are present, but it can be considered that their concentration is rather low compared to some zircon.
To my point of view, despite its attractive vivid-green color, I would not recommend to wear it as a jewellery because of its rather high radioactivity level.
[1] Zircon Visible Spectra - List of Visible Data Files on the Caltech Mineral Spectroscopy Server
[2] Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002, ISBN: 5901837053
[3] Modern Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, 2015, Springer Editor, 2nd Edition, ISBN: 9783319247632

