Yellow and blue flurorite from Valzergues, Aveyron, France
- Details
- Created: Saturday, 05 January 2019 19:58
The two samples of this work (figure 1) have been provided by Spf GEMS, they are an oval yellow and a square blue cabochons. The colors are really typical of the locality.
 Figure 1. The 3.66 ct oval yellow cab and the 2.14 ct square blue
Figure 1. The 3.66 ct oval yellow cab and the 2.14 ct square bluecab of fluorite from Valzergues, Aveyron, France.
| Shape | oval yellow cabochon, square blue cabochon |
| Size | yellow: 11.2 x 8.1 x 4.9 mm, blue: 7.4 x 7.3 x 4.0 mm |
| Color | yellow, blue in daylight and violetish-blue in incandescent light |
| Lustre | sub-vitreous to vitreous |
| Weight | yellow: 3.66 ct, blue: 2.14 ct |
| SG | yellow: 316, blue: 3.19 |
| RI | - |
| DR | - |
| Pleochroism | - |
| Polariscope / Conoscope | stay dark: isotropic |
| SWUV | yellow: chalky-yellow, blue: inert |
| LWUV | yellow: moderate violet, blue: moderate violet |
| Magnetic susceptibility N52 | yellow and blue cab are diamagnetic |
| Chelsea filter | yellow: inert, blue: pinkish-red |
Table 1. Observational and measured properties
Infrared reflectance spectroscopy:
The IR reflectance specta (figure 2) were collected from the cabs' back, the surface being almost flat, it makes the collected area larger than on the top improving the signal. The spectra are characteristic of fluorite with their invariable simple pattern below 600 cm-1.
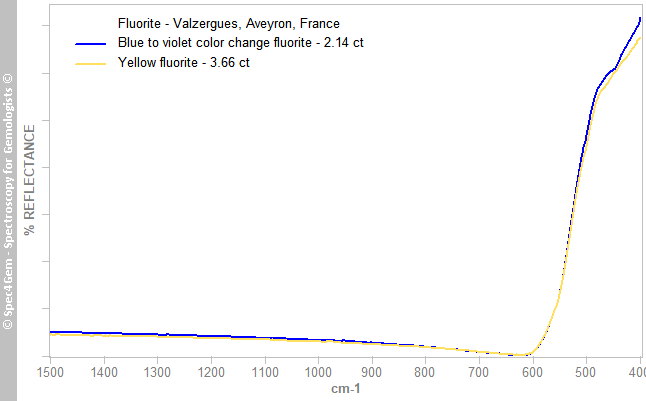 Figure 2. IR reflectance spectra of the yellow fluorite (in yellow) and of the blue flurorite (in blue) from Valzergues.
Figure 2. IR reflectance spectra of the yellow fluorite (in yellow) and of the blue flurorite (in blue) from Valzergues. UV-VIS-NIR spectroscopy:
The UV-Vis-NIR spectra were collected with the path light crossing the cabs from the top to the back, anyhow this does not matter because fluorite material is isotropic.
The spectrum of the yellow fluorite (figure 3) shows a quite complex pattern with a main and large band around 440 nm having a FWHM (full width at half maximum) of about 100 nm. It is decorated by a lot small humps at 403, 419, 436, 551, etc., in fact each of them correspond to a band when all combined (by addition) form the main band. This particular asorption pattern is usually explained by O2- replacing two F- ions. Another significant band is present around 937 nm, it is unexplained at the moment. The large absorption occuring in the blue and the green range of the visible spectrum, it causes the yellow color. One can ask the question, the yellow light is transmitted, but the red also since the absorption is even lower than in yellow, so why the stone is yellow and not orange nor red?
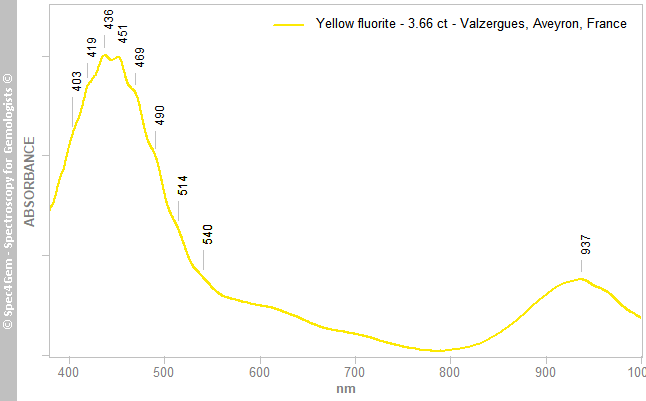 Figure 3. The UV-Vis-NIR spectrum of the yellow fluorite cab shows a large absorption band 'resulting of the multiple combined bands at 403, 419, 436, 451 nm, ... caused by O2- replacing two F- ions. The 937 nm band is unexplained.
Figure 3. The UV-Vis-NIR spectrum of the yellow fluorite cab shows a large absorption band 'resulting of the multiple combined bands at 403, 419, 436, 451 nm, ... caused by O2- replacing two F- ions. The 937 nm band is unexplained. The spectrum of the blue fluorite (figure 4) shows a different pattern of that observed for the yellow one. It has two main gaussian bands at 409 and 580 nm followed by a weak and large band at 920 nm as for the yellow fluorite. The two main bands can have two explanations for the color centers, one is Y2+ substituing for Ca2+ and the other one is O2- and OH- in the structure releasing electron creating a 'light-blue' F-center.
The spectrum pattern creates a transmission window in the blue, blue-green and one in the red, deep-red that explains the blue color of this fluorite but also the color-change from blue to almost purplish-violet that operates if the stone is lit with an incandescent light source.
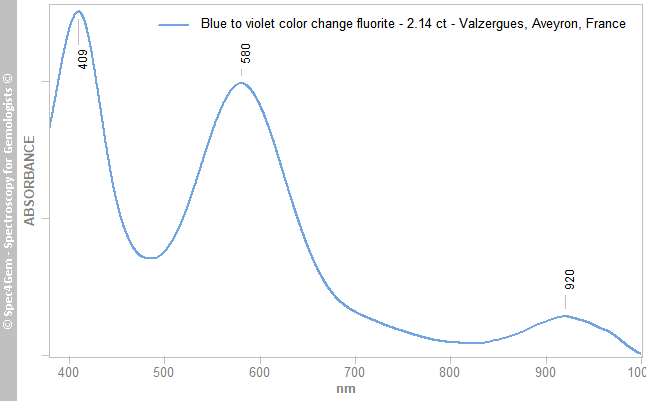 Figure 4. The UV-Vis-NIR spectrum of the blue fluorite cab shows two main gaussian band at 409 and 580 nm known to be caused by Y2+ substituing for Ca2+ and the other one by O2- and OH- releasing electron creating a 'light-blue' F-center.
Figure 4. The UV-Vis-NIR spectrum of the blue fluorite cab shows two main gaussian band at 409 and 580 nm known to be caused by Y2+ substituing for Ca2+ and the other one by O2- and OH- releasing electron creating a 'light-blue' F-center.Photoluminescence spectroscopy:
The photoluminescence spectroscopy has been performed with three sources 405, 377 and 254 nm. The yellow fluorite photoluminescnce spectra (figure 5), all have the same emission feature in the violet-blue around 426-428 nm, the power of the 405 nm laser produces a strong emission truncated at 450 nm by the high-pass filter used to eliminate the laser line. The 405 nm excitation induces a large emission between 500 and 800 nm. This kind of emission is not seen with the two others excitation at 377 and 254 nm. The luminescence with the 254 nm excitation has a singularity with an additional emisssion between 450 and 600 nm.
The 426-428 nm emission is related to Eu2+ occupying two sites producing two emissions, one at 423 and the other one at 430 nm.
The large emission between 500 and 800 nm is related to the commonly called 'ST' luminescence (Singlet-Triplet) caused by organic molecules, often found in mineral of sedimentary origins.
The emission between 450 and 600 nm caused by the 254 nm excitation source is not understood at the moment, it needs further investigation!
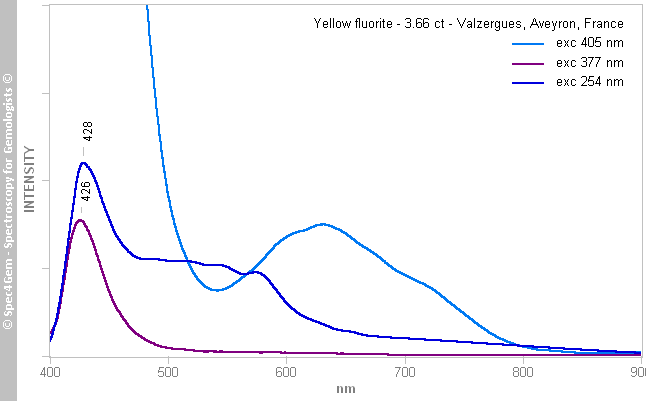 Figure 5. The photoluminescence spectra obtained from the yellow fluorite show the following emissions: 426-428 nm related to Eu2+, between 500 and 800 nm related to the 'ST' luminescence caused by organic molecules, and the ununderstood one between 450 and 600 nm while excited by the 254 nm source.
Figure 5. The photoluminescence spectra obtained from the yellow fluorite show the following emissions: 426-428 nm related to Eu2+, between 500 and 800 nm related to the 'ST' luminescence caused by organic molecules, and the ununderstood one between 450 and 600 nm while excited by the 254 nm source.The blue fluorite photoluminescence spectra (figure 6), as for the yellow fluorite, all have the same emission feature in the violet-blue around 426-428 nm, the power of the 405 nm laser produces a strong emission truncated at 450 nm by the high-pass filter used to eliminate the laser line. Unlike for the yellow fluorite, the 405 nm excitation induces a weak emission centered at 727 nm which is not visible in the 377 and 254 nm spectra, possibly because of the weakness of the later sources.
As for the yellow fluorite, the 426-428 nm emission is related to Eu2+. The 727 nm emission is related to the M-center (= 2F-centers + Na+).
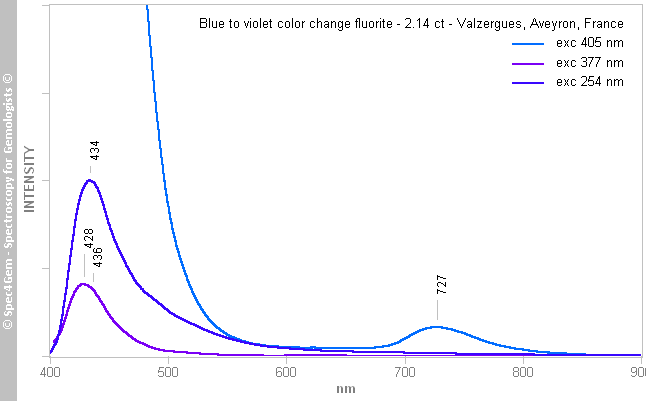 Figure 6. The photoluminescence spectra obtained from the blue fluorite show the following emissions: 426-428 nm related to Eu2+, 727 nm related to the M-center (= 2F-centers + Na+).
Figure 6. The photoluminescence spectra obtained from the blue fluorite show the following emissions: 426-428 nm related to Eu2+, 727 nm related to the M-center (= 2F-centers + Na+).Conclusion:
All data are consistent with fluorites of these colors. No discovery for sure, but documenting such material has been of interest to us because it illustrates what is behind the generality pertaining to fluorite colors.
An interesting improvement would be to study the thermoluminescence on crystals fragments, doing so on the cabs could make the cabs explode!
A picture of crystals with the two colors, the blue surrounding the yellow, and even the light-yellow surrounding the blue surrounding the yellow, the Valzergues locality is known for its three layers (phases) fluorites, indicating a change in the fluids through the crystallisation period.

